Abstract
Background: Activation of extracellular-signal regulated kinase (ERK) is almost universal in histiocytic disorders and is driven by genomic alterations in the mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinases (PI3K) pathways. While BRAFV600E mutations are well described in histiocytic disorders, less is known about BRAF fusions. The goal of our study is to describe the frequency and clinical characteristics of patients harboring BRAF fusions.
Methods: We included patients with a diagnosis of histiocytic disorder seen at our institution from November 2016-June 2021. Only those who had adequate BRAF testing were included. The following were considered adequate BRAF testing: 1) unequivocally positive BRAF V600E immunostain with or without molecular confirmation; 2) successful multigene next generation sequencing (mostly Tempus â or FoundationOne â) if BRAF V600Eimmunostain was equivocal or negative. We also searched the literature for similar cases and compared BRAF fusions found in histiocytic disorders to those found in solid tumors and other hematologic conditions.
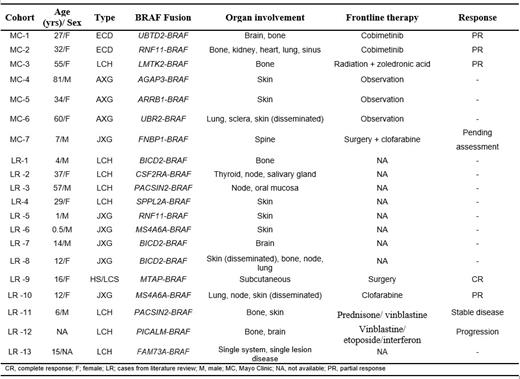
Results: One hundred and twenty-six patients were included in this study. BRAF fusions were detected in 7 (6%) patients. The median follow-up for these patients was 1.4 years (95% CI: 0.4-not reached). The frequency according to disease subtypes is as follows: Erdheim-Chester disease (ECD; 2/46 [4%]), Langerhans cell histiocytosis (LCH; 1/41 [2%]), adult or juvenile xanthogranuloma (AXG/JXG; 4/7 [57%]), and histiocytic or Langerhans cell sarcoma (HS/LCS; 0/9 [0%]). The median age at diagnosis for our study cohort was 34 years (range, 7-81 years) and 5 were females. The clinical characteristics and nature of BRAF fusions of our 7 patients plus additional 13 cases from the literature are shown in Table 1. For the final cohort of 20 patients with BRAF-fusions, the median age was 16 years (range, 0.5-81 years) and most (56%) were females. The distribution by histiocytic subtypes is as follows: AXG/JXG (45%), LCH (40%), ECD (10%), HS/LCS (5%). Two patients received cobimetinib as frontline treatment in our cohort and achieved partial responses. Of these two patients, one completed 12 cycles (12 months) of cobimetinib and had a sustained PR while the other patient received 2 cycles before having to hold therapy due to intolerable adverse effects despite dose reduction. From literature review, another patient (LR-12) received cobimetinib as second line treatment and achieved complete response. We found 15 unique BRAF gene fusions with 4 being recurrent. Except for AGAP3-BRAF, none of these BRAF fusions in histiocytosis has been reported in solid tumors or hematologic conditions to date.
Conclusions: BRAF gene fusions occur rarely (<5%) in histiocytic disorders. The only exception is AXG/JXG where more than half of the patients harbor BRAF gene fusions. These gene fusions are mostly distinct to histiocytosis and not found in solid tumors or other hematologic conditions.
Bennani: Purdue Pharma: Other: Advisory Board; Daichii Sankyo Inc: Other: Advisory Board; Kyowa Kirin: Other: Advisory Board; Vividion: Other: Advisory Board; Kymera: Other: Advisory Board; Verastem: Other: Advisory Board.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal